touchCONGRESS Scanning the horizon of pharmacological treatments for women experiencing vasomotor symptoms during menopause: What’s new in 2022?
Watch this two-part activity exploring the latest data on pharmacological treatments for vasomotor symptoms (VMS) associated with menopause. Filmed following the 2022 North American Menopause Society (NAMS) Annual Meeting and the International Menopause Society (IMS) 18th World Congress on Menopause.
Part 1: Watch menopause expert Prof. Steven Goldstein review key data from the 2022 NAMS Annual Meeting and the IMS 18th World Congress on Menopause Watch Now
Part 2: Choose from leading menopause experts who discuss what these data may mean for clinical practice in the USA Select An Interview
Introduction
Hormone therapy for VMS associated with menopause
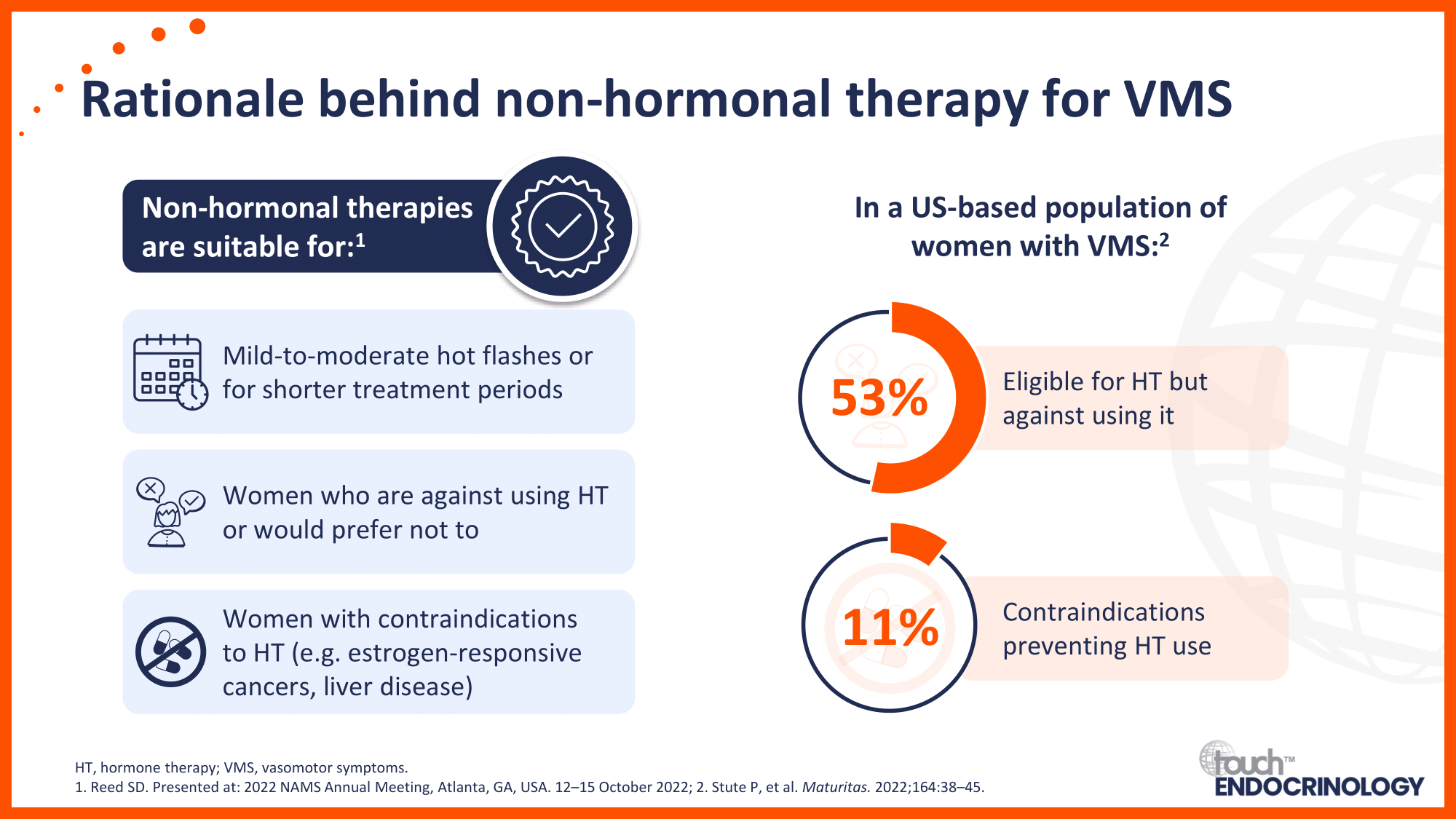
Non-hormonal therapy for VMS associated with menopause
Overview
Watch Prof. Steven Goldstein summarize and share his interpretation of the latest data from the 2022 NAMS Annual Meeting and the IMS 18th World Congress on Menopause on pharmacological treatments for VMS associated with menopause. During the presentation, he considers:
- Hormone therapy for VMS associated with menopause
- Non-hormonal therapy for VMS associated with menopause
Steven R Goldstein is professor of obstetrics and gynaecology at the New York University Grossman School of Medicine in New York City, New York, USA. read more
Prof. Goldstein is a former president of the IMS (2021–2022) and past president and fellow of the American Institute of Ultrasound in Medicine. He is also a past president of the NAMS, past chairman of the American College of Obstetricians and Gynecologists, New York section, and an honorary fellow of the Royal College of Obstetricians and Gynaecologists.
Prof. Goldstein is one of the most highly recognized and regarded individuals in the field of vaginal probe ultrasound worldwide. He has been a guest faculty member, invited speaker, visiting professor or course director over 400 times throughout the USA and the world.
In 2016, Prof. Goldstein received the NAMS/Thomas B. Clarkson Outstanding Clinical and Basic Science Research Award, and in 2019, he received the Joseph H. Holmes Basic Science Pioneer Award from the American Institute of Ultrasound in Medicine. Prof. Goldstein has published seven books on women’s health, and has authored more than 60 chapters and over 140 original research articles.
Prof. Steven Goldstein discloses Advisory board or panel fees from Myovant Sciences and SCYNEXIS (relationships terminated). Consultancy fees from CooperSurgical (relationship terminated) and Cook Medical. Other financial or material support from GE Ultrasound.
Prof. Steven Goldstein considers the latest data from the 2022 NAMS Annual Meeting and the IMS 18th World Congress on Menopause on pharmacological treatments for VMS associated with menopause, including the role of hormone therapy, and how emerging non-hormonal therapies are likely to impact clinical practice and patient outcomes, once approved.
Dr Samuel Lederman considers the latest data from the 2022 NAMS Annual Meeting and the IMS 18th World Congress on Menopause on pharmacological treatments for VMS associated with menopause, including the role of hormone therapy, and how emerging non-hormonal therapies are likely to impact clinical practice and patient outcomes, once approved.
Prof. JoAnn Pinkerton considers the latest data from the 2022 NAMS Annual Meeting and the IMS 18th World Congress on Menopause on pharmacological treatments for VMS associated with menopause, including the role of hormone therapy, and how emerging non-hormonal therapies are likely to impact clinical practice and patient outcomes, once approved.
Please Select A Video:
Overview & Learning Objectives
Overview
In this two-part activity, explore the latest data on pharmacological hormone therapy and non-hormonal therapy for VMS associated with menopause. This activity was filmed following the 2022 NAMS Annual Meeting (12–15 October 2022) and the IMS 18th World Congress on Menopause (26–29 October 2022).
This activity is jointly provided by USF Health and touchIME. read more
Target Audience
This activity has been designed to meet the educational needs of gynaecologists, primary care physicians and endocrinologists involved in the management of menopause.
Disclosures
USF Health adheres to the Standards for Integrity and Independence in Accredited Continuing Education. All individuals in a position to influence content have disclosed to USF Health any financial relationship with an ineligible organization. USF Health has reviewed and mitigated all relevant financial relationships related to the content of the activity. The relevant relationships are listed below. All individuals not listed have no relevant financial relationships.
Faculty
Prof. Steven Goldstein discloses Advisory board or panel fees from Myovant Sciences and SCYNEXIS (relationships terminated). Consultancy fees from CooperSurgical (relationship terminated) and Cook Medical. Other financial or material support from GE Ultrasound.
Dr Samuel Lederman discloses Grants/research support from Aspira, Astellas Pharma, GSK, INOVIO Pharmaceuticals, Janssen Pharmaceuticals, Moderna, Mylan, Myovant Sciences, Parexel, Sebela Pharmaceuticals and Spruce Biosciences. Salary or contractual services from InMode. Speaker’s bureau fees from AbbVie and Mycovia Pharmaceuticals. Stock/shareholder (self-managed) fees from LabConnect.
Dr JoAnn Pinkerton discloses Grants/research support from Bayer.
Content reviewer
Karen Bruder, MD has no financial interests/relationships or affiliations in relation to this activity.
Touch Medical Director
Katrina Lester has no financial interests/relationships or affiliations in relation to this activity.
USF Health Office of Continuing Professional Development and touchIME staff have no financial interests/relationships or affiliations in relation to this activity.
Requirements for Successful Completion
In order to receive credit for this activity, participants must review the content and complete the post-test and evaluation form. Statements of credit are awarded upon successful completion of the post-test and evaluation form.
If you have questions regarding credit please contact cpdsupport@usf.edu.
Accreditations
Physicians
This activity has been planned and implemented in accordance with the accreditation requirements and policies of the Accreditation Council for Continuing Medical Education (ACCME) through a joint providership of USF Health and touchIME. USF Health is accredited by the ACCME to provide continuing medical education for physicians.
USF Health designates this enduring material for a maximum of 1.0 AMA PRA Category 1 CreditTM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
The European Union of Medical Specialists (UEMS) – European Accreditation Council for Continuing Medical Education (EACCME) has an agreement of mutual recognition of continuing medical education (CME) credit with the American Medical Association (AMA). European physicians interested in converting AMA PRA Category 1 CreditTM into European CME credit (ECMEC) should contact the UEMS (www.uems.eu).
Advanced Practice Providers
Physician Assistants may claim a maximum of 1.0 Category 1 credits for completing this activity. NCCPA accepts AMA PRA Category 1 CreditTM from organizations accredited by ACCME or a recognized state medical society.
The AANPCP accepts certificates of participation for educational activities approved for AMA PRA Category 1 CreditTM by ACCME-accredited providers. APRNs who participate will receive a certificate of completion commensurate with the extent of their participation.
Date of original release: 12 December 2022. Date credits expire: 12 December 2023.
If you have any questions regarding credit please contact cpdsupport@usf.edu.
Learning Objectives
After watching this activity, participants should be better able to:
- Describe the benefits and risks of hormone therapy for vasomotor symptoms associated with menopause
- Outline the rationale for non-hormonal therapy and mechanisms of action of emerging options for the treatment of vasomotor symptoms associated
with menopause - Recall the latest data for emerging non-hormonal therapies for the treatment of vasomotor symptoms associated with menopause

REGISTER NOW FOR FREE ACCESS TO
- 1000+ topical and insightful peer-reviewed journal articles
- 100+ hours of bite-sized congress highlights
- 8 major therapy areas packed with the latest scientific advances
- 150+ specialties offering learn-on-the-go medical education
- + Concise email updates and newsletters so you never miss out